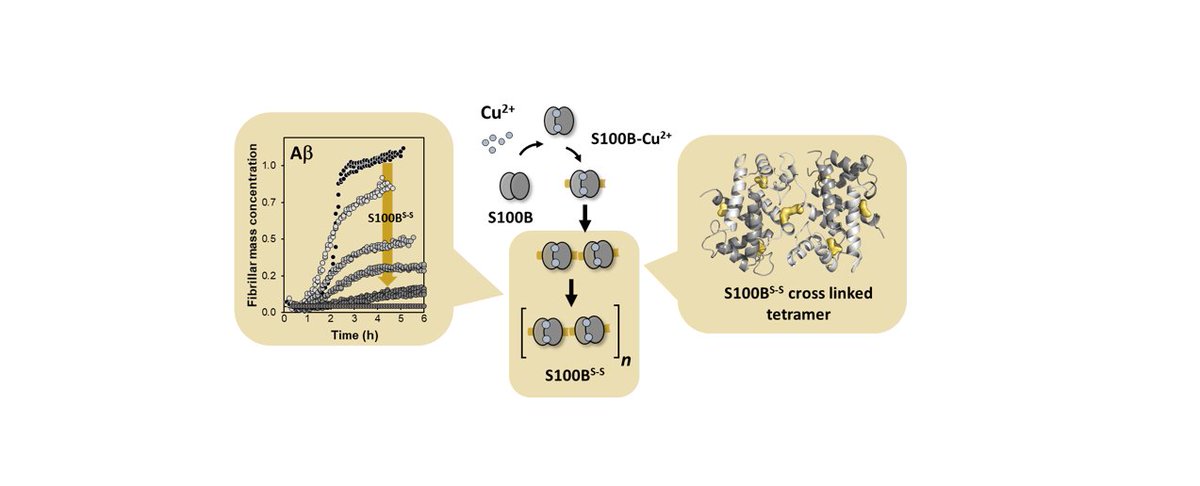
Now available: Cu(II)-binding to S100B triggers polymerization of disulfide cross-linked tetramers with enhanced chaperone activity against amyloid-β aggregation - just published in Chemical Communications @ChemCommun https://pubs.rsc.org/en/content/articlelanding/2020/cc/d0cc06842j">https://pubs.rsc.org/en/conten...
S100B is an extracellular protein implicated in Alzheimer’s Disease and a supressor of amyloid-β aggregation. Herein we report a mechanism tying Cu2+ binding to a change in assembly state yielding disulfide cross-linked oligomers with higher anti-aggregation activity.
This chemical control of chaperone function illustrates a regulatory process relevant under metal and proteostasis dysfunction as in neurodegeneration.
This was a collaborative study that involved colleagues with different expertise, to which we are very grateful for their input into our work @FC_UL @i3S_UPorto @UniHohenheim

 Read on Twitter
Read on Twitter