THREAD: Encouraging late-stage trial results from @pfizer and @moderna_tx's vaccines  will likely increase confidence that more shots will work and that the world may soon find a way to get #Covid19 under control
will likely increase confidence that more shots will work and that the world may soon find a way to get #Covid19 under control
Here's how the two vaccines stack up:
https://trib.al/DhEf0go
 will likely increase confidence that more shots will work and that the world may soon find a way to get #Covid19 under control
will likely increase confidence that more shots will work and that the world may soon find a way to get #Covid19 under controlHere's how the two vaccines stack up:
https://trib.al/DhEf0go
The results:
 Moderna's vaccine was 94.5% effective, with short-lived side effects and no significant safety concerns
Moderna's vaccine was 94.5% effective, with short-lived side effects and no significant safety concerns
 Pfizer and BioNTech's shot was found to be more than 90% effective
Pfizer and BioNTech's shot was found to be more than 90% effective
https://trib.al/DhEf0go
 Moderna's vaccine was 94.5% effective, with short-lived side effects and no significant safety concerns
Moderna's vaccine was 94.5% effective, with short-lived side effects and no significant safety concerns  Pfizer and BioNTech's shot was found to be more than 90% effective
Pfizer and BioNTech's shot was found to be more than 90% effective https://trib.al/DhEf0go
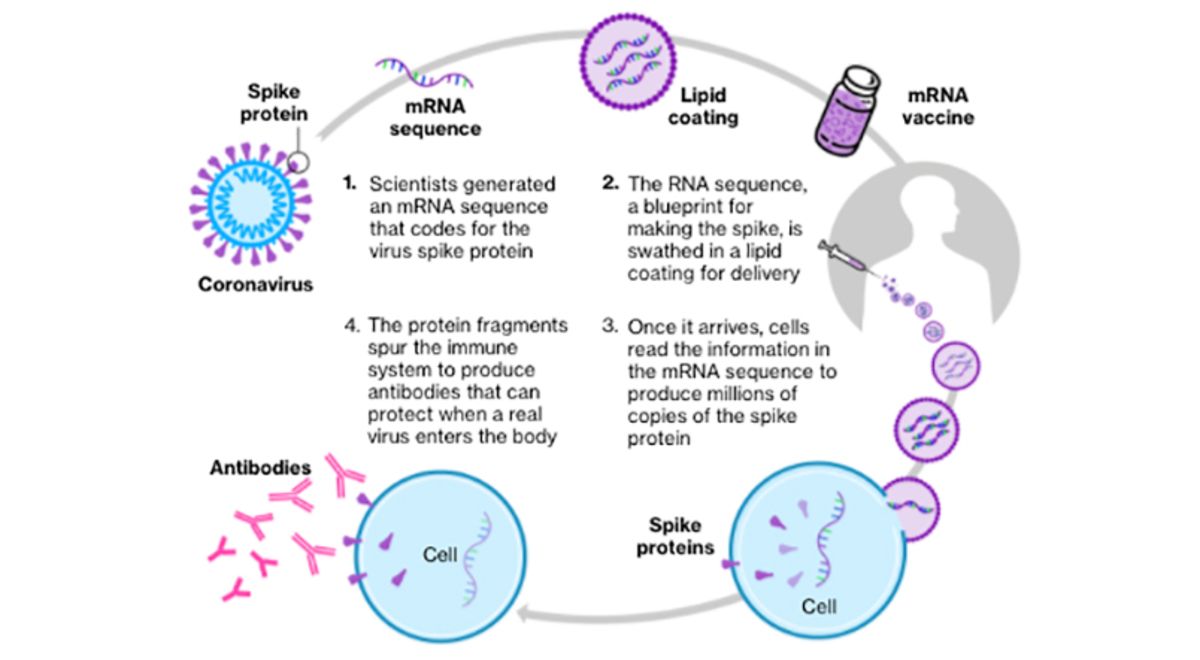
 Both shots use messenger RNA
Both shots use messenger RNA  mRNA never been used before to develop an approved vaccine
mRNA never been used before to develop an approved vaccine The vaccines instruct cells to make copies of the spike protein of the coronavirus, stimulating the creation of protective antibodies
The vaccines instruct cells to make copies of the spike protein of the coronavirus, stimulating the creation of protective antibodies https://trib.al/DhEf0go
The money:
 Moderna got $955 million from the U.S. Operation Warp Speed program.
Moderna got $955 million from the U.S. Operation Warp Speed program.
 Pfizer said it didn’t receive any federal funding to develop its vaccine, though BioNTech got as much as $444 million in German government assistance
Pfizer said it didn’t receive any federal funding to develop its vaccine, though BioNTech got as much as $444 million in German government assistance
https://trib.al/DhEf0go
 Moderna got $955 million from the U.S. Operation Warp Speed program.
Moderna got $955 million from the U.S. Operation Warp Speed program.  Pfizer said it didn’t receive any federal funding to develop its vaccine, though BioNTech got as much as $444 million in German government assistance
Pfizer said it didn’t receive any federal funding to develop its vaccine, though BioNTech got as much as $444 million in German government assistance https://trib.al/DhEf0go
 Pfizer’s vaccine must be stored ultra-cold until a few days before it is used, but can be kept at refrigerator temperatures for up to 5 days.
Pfizer’s vaccine must be stored ultra-cold until a few days before it is used, but can be kept at refrigerator temperatures for up to 5 days.  Moderna said its vaccine is stable at refrigerator temperatures for 30 days
Moderna said its vaccine is stable at refrigerator temperatures for 30 days https://trib.al/DhEf0go
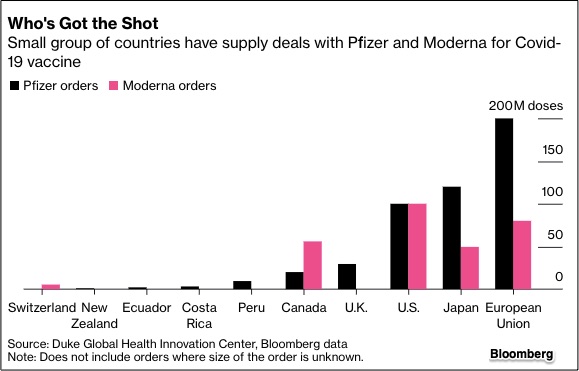
Moderna has agreements to supply 100 million doses to the U.S. and 80 million to the European Union, among others.
 The U.K. said it's negotiating with the company but doses wouldn’t become available in the country until next spring https://trib.al/DhEf0go
The U.K. said it's negotiating with the company but doses wouldn’t become available in the country until next spring https://trib.al/DhEf0go
 The U.K. said it's negotiating with the company but doses wouldn’t become available in the country until next spring https://trib.al/DhEf0go
The U.K. said it's negotiating with the company but doses wouldn’t become available in the country until next spring https://trib.al/DhEf0go
Moderna and Pfizer are expected to seek emergency-use authorization from @US_FDA
 Moderna said it could seek clearance from regulators in the coming weeks
Moderna said it could seek clearance from regulators in the coming weeks
 Pfizer could apply for an authorization in the U.S. this month
Pfizer could apply for an authorization in the U.S. this month
https://trib.al/DhEf0go
 Moderna said it could seek clearance from regulators in the coming weeks
Moderna said it could seek clearance from regulators in the coming weeks  Pfizer could apply for an authorization in the U.S. this month
Pfizer could apply for an authorization in the U.S. this monthhttps://trib.al/DhEf0go

 Read on Twitter
Read on Twitter